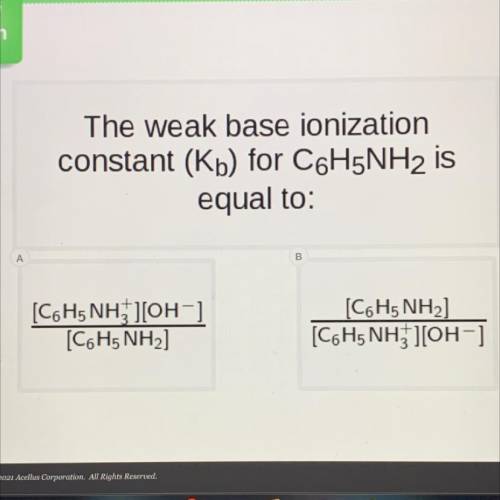
The weak base ionization
constant (Kb) for C6H5NH2 is
equal to:
...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Questions



Mathematics, 07.04.2021 20:00

Mathematics, 07.04.2021 20:00




English, 07.04.2021 20:00


Mathematics, 07.04.2021 20:00


Mathematics, 07.04.2021 20:00

Mathematics, 07.04.2021 20:00





Chemistry, 07.04.2021 20:00


Mathematics, 07.04.2021 20:00