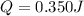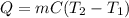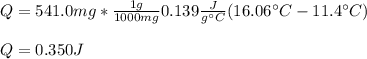Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3


Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
Calculate the energy required to heat 541.0mg of mercury from 11.4°C to 16.6°C. Assume the specifi...
Questions

Biology, 21.07.2019 19:40

Mathematics, 21.07.2019 19:40


Mathematics, 21.07.2019 19:40


Biology, 21.07.2019 19:40

Mathematics, 21.07.2019 19:40

Mathematics, 21.07.2019 19:40

Mathematics, 21.07.2019 19:40

English, 21.07.2019 19:40


Mathematics, 21.07.2019 19:40

Mathematics, 21.07.2019 19:40




Advanced Placement (AP), 21.07.2019 19:40


Mathematics, 21.07.2019 19:40

Mathematics, 21.07.2019 19:40