
Chemistry, 13.01.2021 08:00 lildee16lildee
(04.07 HC)
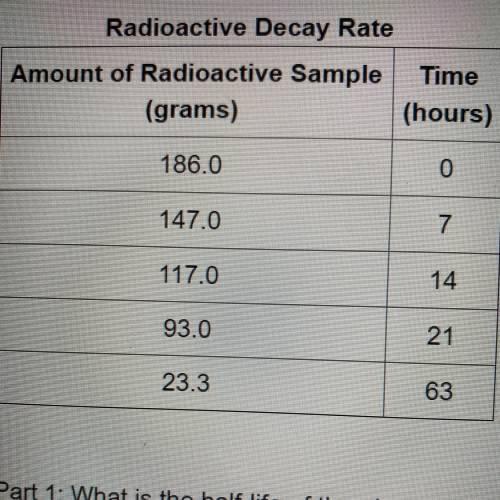
The table shows the amount of radioactive element remaining in a sample over a period of time,
Radioactive Decay Rate
Amount of Radioactive Sample Time
(grams)
(hours)
186.0
0
147.0
7
117.0
14
93.0
21
23.3
63
Part 1: What is the half-life of the element? Explain how you determined this
Part 2: How long would it take 320 g of the sample to decay to 2.5 grams? Show your work or explain your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
(04.07 HC)
The table shows the amount of radioactive element remaining in a sample over a period of...
Questions






Mathematics, 12.03.2020 20:21


Business, 12.03.2020 20:21




Mathematics, 12.03.2020 20:21




History, 12.03.2020 20:21


Computers and Technology, 12.03.2020 20:21




