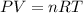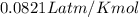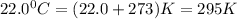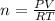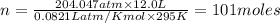Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
an oxygen tank used for scuba diving has. avolume of 12.0 L .If the pressure of the tank is known to...
Questions


Mathematics, 14.07.2021 08:50

Mathematics, 14.07.2021 08:50


Mathematics, 14.07.2021 08:50

Mathematics, 14.07.2021 08:50

Mathematics, 14.07.2021 08:50






Social Studies, 14.07.2021 08:50

Mathematics, 14.07.2021 08:50

History, 14.07.2021 08:50



English, 14.07.2021 08:50

Mathematics, 14.07.2021 08:50

Mathematics, 14.07.2021 08:50