Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

You know the right answer?
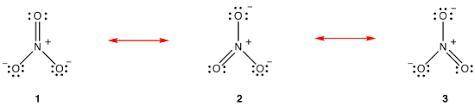
Draw the Lewis structures for three resonance forms of the nitrate ion, NO−3 . Include electron lone...
Questions



Mathematics, 30.04.2021 03:50




History, 30.04.2021 03:50

Mathematics, 30.04.2021 03:50




Mathematics, 30.04.2021 03:50

History, 30.04.2021 03:50

History, 30.04.2021 03:50

Mathematics, 30.04.2021 03:50


English, 30.04.2021 03:50

Mathematics, 30.04.2021 03:50

Mathematics, 30.04.2021 03:50

Mathematics, 30.04.2021 03:50