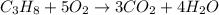Chemistry, 13.01.2021 18:30 zariasimone2
Propane is often used in stoves and cooktops as a source of heat. The combustion of propane yields water and carbon
unbalanced chemical reaction is represented by the ball-and-stick diagrama shown.
How many molecules of carbon dioxide and water are produced during the combustion of propane? Move numbers to
the equation and show that mass is conserved.
+
+
Propane molecule
Oxygen molecules
Carbon dioxide molecule
Water molecule
Legend
Hydrogen
atom
Oxygen
atom
Carbon
atom
2
3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3


Chemistry, 23.06.2019 10:30
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1
You know the right answer?
Propane is often used in stoves and cooktops as a source of heat. The combustion of propane yields w...
Questions

History, 03.05.2020 13:01

English, 03.05.2020 13:01

Mathematics, 03.05.2020 13:01



Mathematics, 03.05.2020 13:01

Mathematics, 03.05.2020 13:01




Business, 03.05.2020 13:01

Mathematics, 03.05.2020 13:01



Mathematics, 03.05.2020 13:01



Mathematics, 03.05.2020 13:01