
HELP ASAPPP
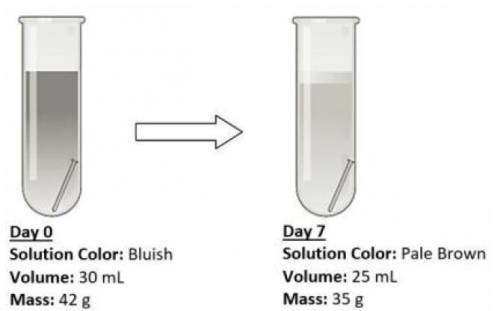
An iron nail was placed in an open test tube containing some salt solution, and then left for a week for observations. The experimental setup and observations are shown below.
Which of the following best demonstrates that a chemical change has taken place?
The salt solution was discolored after a week.
The volume of the salt solution has decreased.
The mass of the salt solution, beaker and nails decreased.
The iron nail is still undissolved in the solution after a week.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
HELP ASAPPP
An iron nail was placed in an open test tube containing some salt solution, and then le...
Questions




Mathematics, 19.02.2020 02:55

History, 19.02.2020 02:55








Mathematics, 19.02.2020 02:55





History, 19.02.2020 02:55

Mathematics, 19.02.2020 02:55

Mathematics, 19.02.2020 02:55



