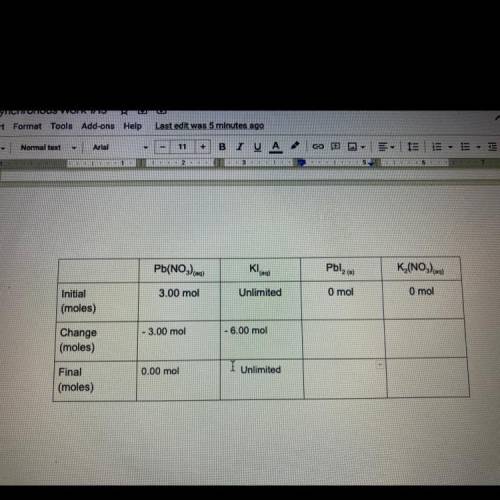
Task 4
3.00 moles of lead nitrate and excess potassium iodidq are combined in water to
form l...

Task 4
3.00 moles of lead nitrate and excess potassium iodidq are combined in water to
form lead iodide and potassium nitrate. The reaction is described by the following
chemical equation:
Pb(NO3) eq) + 2Kleg) Pblzco + K (NO)
Fill out the following table to find out how many moles of products are formed,
and how many of the reactants remain after the reaction has taken place. The boxes in
the final row are all mole to mole conversions, and can be found by using your molar
ratios and the moles of lead nitrate at the beginning of the reaction. It is your choice
how you will arrange these two numbers. If this problem becomes confusing, look back
to task 3, It's the same problem!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

You know the right answer?
Questions

Mathematics, 14.11.2021 23:30

Mathematics, 14.11.2021 23:30



Physics, 14.11.2021 23:30




Physics, 14.11.2021 23:30


Advanced Placement (AP), 14.11.2021 23:30

Mathematics, 14.11.2021 23:30



Biology, 14.11.2021 23:30

Spanish, 14.11.2021 23:30

English, 14.11.2021 23:30



Mathematics, 14.11.2021 23:30




