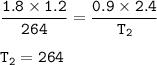Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Which is the most likely way an automotive engineer would use chemistry
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
A gas has 1.2 L of volume, 1.8 atm of pressure and 264 K temperature. If both the
volume was double...
Questions

Mathematics, 23.06.2020 10:57

Advanced Placement (AP), 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Spanish, 23.06.2020 10:57

History, 23.06.2020 10:57

World Languages, 23.06.2020 10:57

History, 23.06.2020 10:57



Mathematics, 23.06.2020 10:57


Mathematics, 23.06.2020 10:57

Physics, 23.06.2020 10:57


Geography, 23.06.2020 10:57