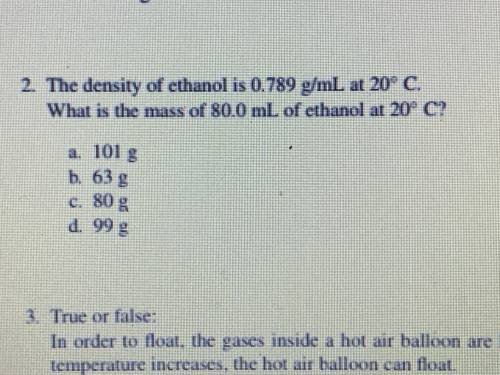
The density of ethanol is 0.789 g/mL at 20 c
what is the mass of 80.0 mL of ethanol at 2...

Chemistry, 13.01.2021 22:40 jakeyywashere
The density of ethanol is 0.789 g/mL at 20 c
what is the mass of 80.0 mL of ethanol at 20 c

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3
You know the right answer?
Questions




Mathematics, 25.02.2022 05:50

English, 25.02.2022 05:50


Mathematics, 25.02.2022 05:50

Physics, 25.02.2022 05:50

English, 25.02.2022 05:50

English, 25.02.2022 06:00


Social Studies, 25.02.2022 06:00


Social Studies, 25.02.2022 06:00



Business, 25.02.2022 06:00

Mathematics, 25.02.2022 06:00


English, 25.02.2022 06:00



