5 of 20
09
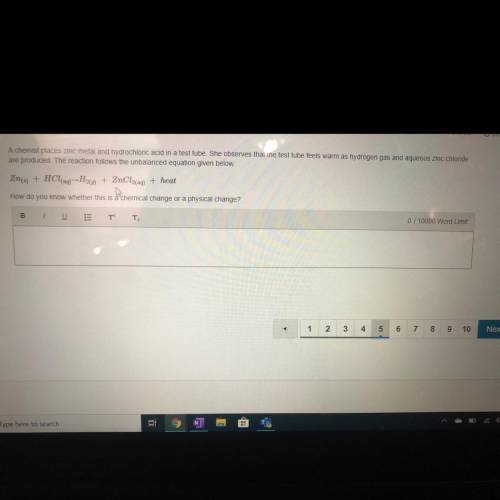
A chemist places zinc metal and hydrochloric acid in a test tube. She observes tha...

Chemistry, 14.01.2021 01:00 uchechukwueigwe
5 of 20
09
A chemist places zinc metal and hydrochloric acid in a test tube. She observes that the test tube feels warm as hydrogen gas and aqueous zinc chloride
are produced. The reaction follows the unbalanced equation given below.
Zn(s) + HCl(aq) - H20) + ZnCl2(aq) + heat
How do you know whether this is a chemical ct ge or a ysical change?
B
U
T
T:
0 / 10000 Word Limit

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3


Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
Questions

Biology, 07.04.2021 22:50



Mathematics, 07.04.2021 22:50



Computers and Technology, 07.04.2021 22:50

Social Studies, 07.04.2021 22:50



Mathematics, 07.04.2021 22:50


Mathematics, 07.04.2021 22:50

Mathematics, 07.04.2021 22:50

Biology, 07.04.2021 22:50


Mathematics, 07.04.2021 22:50

Social Studies, 07.04.2021 22:50

Biology, 07.04.2021 22:50

Mathematics, 07.04.2021 22:50



