
Chemistry, 14.01.2021 01:00 mocheal8216
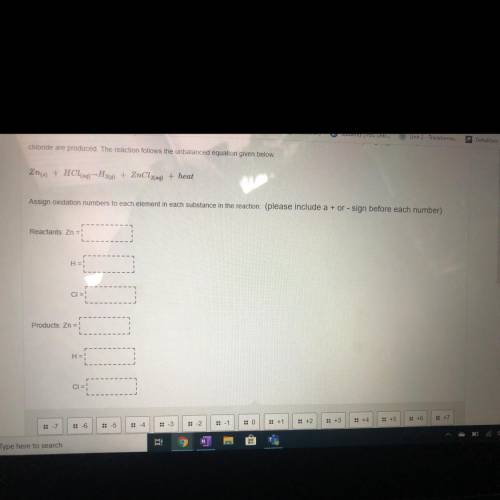
Chloride are produced. The reaction follows the unbalanced equation given below.
Zn(s) + HCl(a) --H26) + ZnCl2(aq) + heat
Assign oxidation numbers to each element in each substance in the reaction (please include a + or - sign before each number)
Reactants: Zn = !
H =
CIE:
Products: Zn =
H = !
CI=
:: +3
: 0
.: +5
:: -7
:: -5
:: -2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this solution?
Answers: 1

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1
You know the right answer?
Chloride are produced. The reaction follows the unbalanced equation given below.
Zn(s) + HCl(a) --H...
Questions


Mathematics, 26.06.2019 04:40

Physics, 26.06.2019 04:40


Physics, 26.06.2019 04:40


Geography, 26.06.2019 04:40

Geography, 26.06.2019 04:40

Geography, 26.06.2019 04:40


Biology, 26.06.2019 04:40

Biology, 26.06.2019 04:40











