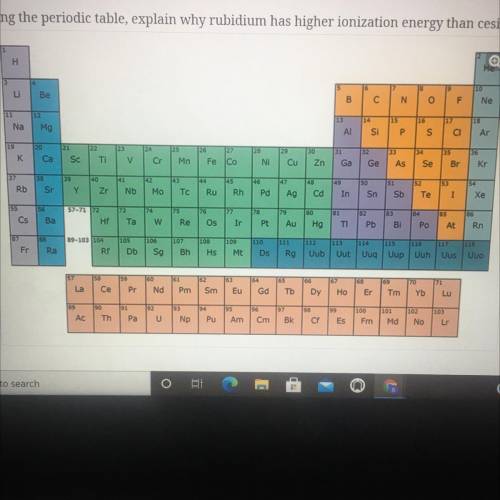
Using the periodic table, explain why rubidium has higher ionization energy than cesium.
...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

You know the right answer?
Questions

Mathematics, 29.10.2020 05:50

Mathematics, 29.10.2020 05:50

Mathematics, 29.10.2020 05:50

Chemistry, 29.10.2020 05:50


Mathematics, 29.10.2020 05:50

Mathematics, 29.10.2020 05:50


Spanish, 29.10.2020 05:50

Social Studies, 29.10.2020 05:50

Chemistry, 29.10.2020 05:50

Mathematics, 29.10.2020 05:50

Mathematics, 29.10.2020 05:50

Biology, 29.10.2020 05:50


Mathematics, 29.10.2020 05:50



Social Studies, 29.10.2020 06:00