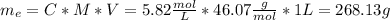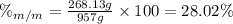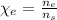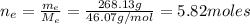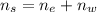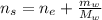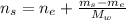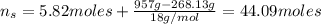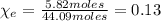Chemistry, 14.01.2021 17:00 aidengalvin20
An aqueous solution of ethanol, CH3CH2OH, has a concentration of 5.82 mol/L and has a density of 0.957 g/mL. What are the mass percent and mole fraction of CH3CH2OH in this solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
An aqueous solution of ethanol, CH3CH2OH, has a concentration of 5.82 mol/L and has a density of 0.9...
Questions


Mathematics, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01



Arts, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01



Mathematics, 18.10.2020 06:01

English, 18.10.2020 06:01

Social Studies, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01



Business, 18.10.2020 06:01

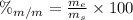
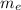 is the mass of ethanol
is the mass of ethanol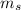 is the mass of the solution
is the mass of the solution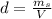
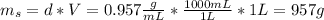
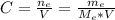
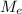 : is the molar mass of ethanol = 46.07 g/mol
: is the molar mass of ethanol = 46.07 g/mol 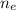 : is the number of moles of ethanol = m/M
: is the number of moles of ethanol = m/M