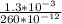Chemistry, 14.01.2021 17:00 terrenevans4156
The radius of a vanadium atom is 130 pm. How many vanadium atoms would have to be laid side by side to span a distance of 1.30 mm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

You know the right answer?
The radius of a vanadium atom is 130 pm. How many vanadium atoms would have to be laid side by side...
Questions


Social Studies, 05.11.2020 17:20



Mathematics, 05.11.2020 17:20




Mathematics, 05.11.2020 17:20


Mathematics, 05.11.2020 17:20


Mathematics, 05.11.2020 17:20

Mathematics, 05.11.2020 17:20



Computers and Technology, 05.11.2020 17:20



 m
m m,
m,