
Chemistry, 14.01.2021 18:10 smallblueberry
i) Mass of CO2 formed = 2.241 g Molar mass of CO2 = Atomic mass of C + 2 (Atomic mass of O) = 12 + 2 (16) g/mol = 44 g/mol Thus, moles of carbon dioxide formed

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2


You know the right answer?
i) Mass of CO2 formed = 2.241 g Molar mass of CO2 = Atomic mass of C + 2 (Atomic mass of O) = 12 + 2...
Questions

Mathematics, 30.05.2020 04:57




Social Studies, 30.05.2020 04:57


Mathematics, 30.05.2020 04:57

Mathematics, 30.05.2020 04:57

History, 30.05.2020 04:57





Biology, 30.05.2020 04:57

Spanish, 30.05.2020 04:58

Mathematics, 30.05.2020 04:58




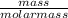
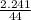 = 0.05mole
= 0.05mole

