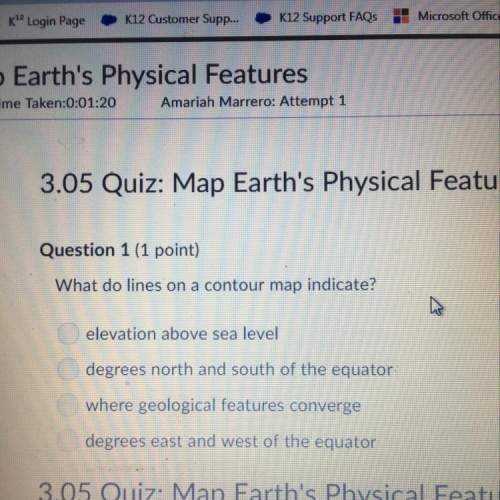
Chemistry, 14.01.2021 19:40 turtlesage21
In an experiment, zinc chlorate decomposed according to the following chemical equation,
Zn(CIO3)2 -- ZnCl2 + O2
(Molar mass of Zn(CIO3)2 = 232.29 g/mol; ZnCl2 = 136.286 g/mol O2 = 31.998 g/mol)
If the mass of zinc chlorate was 150 grams, which of the following calculations can be used to determine the mass of oxygen gas formed?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
In an experiment, zinc chlorate decomposed according to the following chemical equation,
Zn(CIO3)2...
Questions

Computers and Technology, 01.12.2021 03:30


Social Studies, 01.12.2021 03:30

Social Studies, 01.12.2021 03:30

Mathematics, 01.12.2021 03:30

Mathematics, 01.12.2021 03:30




Mathematics, 01.12.2021 03:30

Social Studies, 01.12.2021 03:30








Geography, 01.12.2021 03:30

Mathematics, 01.12.2021 03:30