
Chemistry, 14.01.2021 23:20 Isaacochoa780
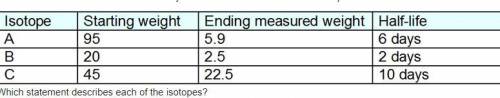
This chart show the amount of radioactivity measured from three unknown isotopes.
Which statement describes each of the isotopes?
Isotope A was measured at day 24, Isotope B was measured at day 6, and Isotope C was measured at day 10.
Isotope A was measured at day 18, Isotope B was measured at day 4, and Isotope C was measured at day 5.
Isotope A was measured at day 24, Isotope B was measured at day 8, and Isotope C was measured at day 20.
Isotope A was measured at day 30, Isotope B was measured at day 6, and Isotope C was measured at day 10.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 08:30
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1
You know the right answer?
This chart show the amount of radioactivity measured from three unknown isotopes.
Which statement d...
Questions

Chemistry, 24.10.2021 14:00

Mathematics, 24.10.2021 14:00






History, 24.10.2021 14:00

Mathematics, 24.10.2021 14:00


Computers and Technology, 24.10.2021 14:00

English, 24.10.2021 14:00


Mathematics, 24.10.2021 14:00

English, 24.10.2021 14:00



History, 24.10.2021 14:00




