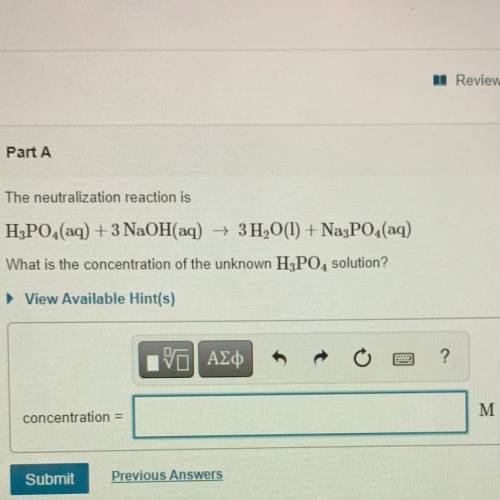
The neutralization reaction is
H3PO4(aq) + 3 NaOH(aq) + 3H2O(l) + Na3PO4(aq)
What is the conc...

Chemistry, 15.01.2021 07:00 SauceyNaee
The neutralization reaction is
H3PO4(aq) + 3 NaOH(aq) + 3H2O(l) + Na3PO4(aq)
What is the concentration of the unknown H3PO4 solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Questions

Spanish, 17.06.2021 15:20


Spanish, 17.06.2021 15:20




French, 17.06.2021 15:20

Arts, 17.06.2021 15:20


Mathematics, 17.06.2021 15:30

History, 17.06.2021 15:30

Mathematics, 17.06.2021 15:30



History, 17.06.2021 15:30

Mathematics, 17.06.2021 15:30



Mathematics, 17.06.2021 15:30

English, 17.06.2021 15:30



