
Chemistry, 15.01.2021 14:00 jacobbrandon2002
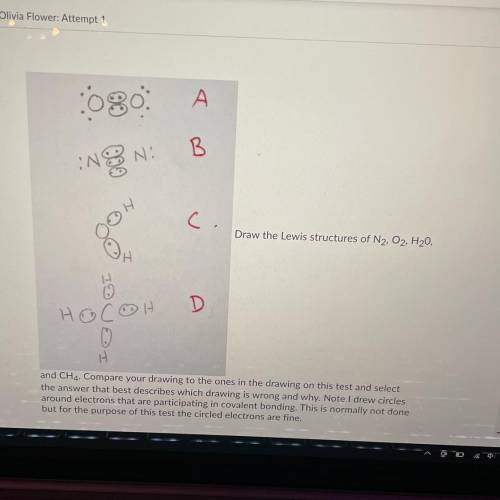
Draw the Lewis structures of N2, O2, H20, and CH4
Compare your drawing to the ones in the drawing on this test and select the answer that best describes which drawing is wrong and why.
A: O2 is wrong because it shows the electrons at a 45 degree angle to the Oxygen atoms
B: N2 is wrong because it shows a triple bond
C: H2O is wrong because it is missing 4 valence electrons
D: CH4 is wrong because the bonds are supposed to be bent at 109.5 degrees

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

You know the right answer?
Draw the Lewis structures of N2, O2, H20, and CH4
Compare your drawing to the ones in the drawing o...
Questions










Chemistry, 08.04.2020 02:11



English, 08.04.2020 02:11






Mathematics, 08.04.2020 02:11



