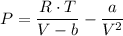A chemist is studying the properties of a gas under various conditions. He observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. When the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal gas. Explain these observations

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1

Chemistry, 23.06.2019 15:30
If the theoretical yield of a reaction is 26.0 grams and you actually recovered 22.0 grams, what is the percent yield?
Answers: 2

Chemistry, 23.06.2019 16:00
When electrons are removed from the outermost shell of a calcium atom, the atom becomes an anion that has a larger radius than the atom. an anion that has a smaller radius than the atom. a cation that has a larger radius than the atom. a cation that has a smaller radius than the atom.
Answers: 3
You know the right answer?
A chemist is studying the properties of a gas under various conditions. He observes that when the ga...
Questions








Biology, 10.08.2021 03:00


Advanced Placement (AP), 10.08.2021 03:00








Mathematics, 10.08.2021 03:10

Advanced Placement (AP), 10.08.2021 03:10