Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
You know the right answer?
Thermodynamics and Q
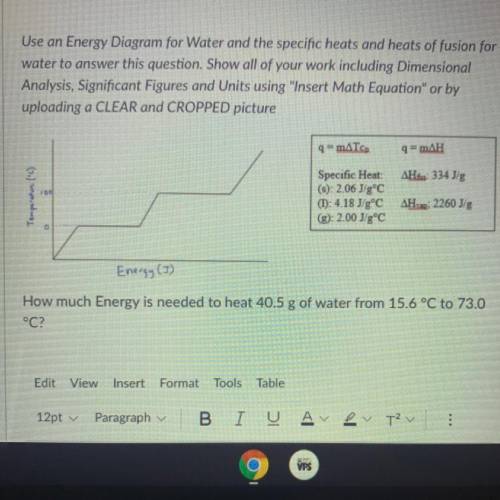
How much energy is needed to heat 40.5g of water from 15.6°C to 73.0°C <...
How much energy is needed to heat 40.5g of water from 15.6°C to 73.0°C <...
Questions

English, 11.03.2021 18:30

Mathematics, 11.03.2021 18:30

Mathematics, 11.03.2021 18:30

Mathematics, 11.03.2021 18:30

Mathematics, 11.03.2021 18:30

Biology, 11.03.2021 18:30


Mathematics, 11.03.2021 18:30




Mathematics, 11.03.2021 18:30

History, 11.03.2021 18:30


Mathematics, 11.03.2021 18:30


English, 11.03.2021 18:30



Mathematics, 11.03.2021 18:30