How do the percent compositions for C3H6 and C4H7 compare?
A. They are the same
B. C4H8 has a...

Chemistry, 16.01.2021 07:00 tiffanyrhoda
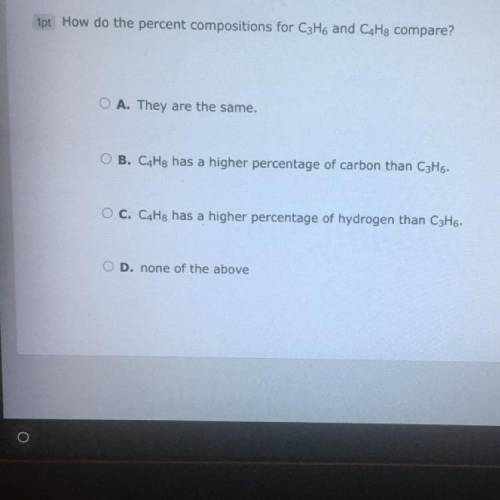
How do the percent compositions for C3H6 and C4H7 compare?
A. They are the same
B. C4H8 has a higher percentage of carbon than C3H6.
C. C4H8 has a higher percentage of hydrogen than C3H6.
D. none of the above

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Questions





English, 13.04.2021 20:00

History, 13.04.2021 20:00

Mathematics, 13.04.2021 20:00


Social Studies, 13.04.2021 20:00

Spanish, 13.04.2021 20:00


English, 13.04.2021 20:00

Mathematics, 13.04.2021 20:00









