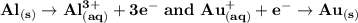Chemistry, 16.01.2021 08:20 skdfnkjsdfh
What are the half-reactions for a galvanic cell with aluminum and gold
electrodes?


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 17:50
The reaction of sodium hydrogen carbonate and acetic acid is described above. using the equation below, identify the proper coefficients for the balanced equation that upholds the law of conversation of mass
Answers: 3
You know the right answer?
What are the half-reactions for a galvanic cell with aluminum and gold
electrodes?
...
electrodes?
...
Questions

Mathematics, 11.01.2021 20:10


Chemistry, 11.01.2021 20:10

World Languages, 11.01.2021 20:10

Mathematics, 11.01.2021 20:10


Mathematics, 11.01.2021 20:10

Mathematics, 11.01.2021 20:10


Mathematics, 11.01.2021 20:10

Mathematics, 11.01.2021 20:10




English, 11.01.2021 20:10



Mathematics, 11.01.2021 20:10

Mathematics, 11.01.2021 20:10

History, 11.01.2021 20:10