
Chemistry, 16.01.2021 14:40 jakeyywashere
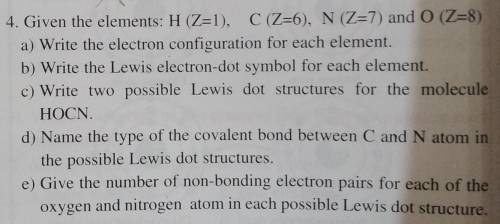
4. Given the elements: H (Z=1), C (Z=6), N (Z=7) and 0 (Z=8)
a) Write the electron configuration for each element.
b) Write the Lewis electron-dot symbol for each element.
c) Write two possible Lewis dot structures for the molecule
HOCN.
d) Name the type of the covalent bond between C and N atom in
the possible Lewis dot structures.
e) Give the number of non-bonding electron pairs for each of the
oxygen and nitrogen atom in each possible Lewis dot structure

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2


Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 13:30
What is one measurement needed to calculate the speed of an object?
Answers: 1
You know the right answer?
4. Given the elements: H (Z=1), C (Z=6), N (Z=7) and 0 (Z=8)
a) Write the electron configuration fo...
Questions


Mathematics, 22.08.2020 20:01





Computers and Technology, 22.08.2020 20:01


Mathematics, 22.08.2020 20:01














