
Chemistry, 16.01.2021 23:40 dewaynesmith46
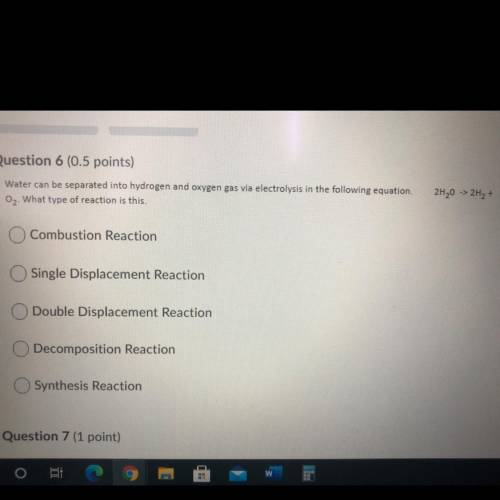
Water can be separated into hydrogen and oxygen gas via electrolysis in the following equation. 2H20 -> 2H2 +O2 What type of reaction is this.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
Water can be separated into hydrogen and oxygen gas via electrolysis in the following equation. 2H20...
Questions

Spanish, 13.02.2021 02:30

Mathematics, 13.02.2021 02:30



Mathematics, 13.02.2021 02:30

Mathematics, 13.02.2021 02:30

Mathematics, 13.02.2021 02:30



Mathematics, 13.02.2021 02:30

Spanish, 13.02.2021 02:30

Physics, 13.02.2021 02:30


Mathematics, 13.02.2021 02:30

Social Studies, 13.02.2021 02:30

Chemistry, 13.02.2021 02:30


Mathematics, 13.02.2021 02:30

Mathematics, 13.02.2021 02:30



