Chemistry, 16.01.2021 23:50 robertjoy19
A sample of carbon dioxide gas at a pressure of 891 mm Hg and a temperature of 84 C occupies a volume of 5.61 L. If the gas is cooled at constant pressure to a temperature of 40 C, the volume of the gas will be __ L.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
A sample of carbon dioxide gas at a pressure of 891 mm Hg and a temperature of 84 C occupies a volum...
Questions

English, 26.12.2021 01:00



English, 26.12.2021 01:00


Social Studies, 26.12.2021 01:00



Biology, 26.12.2021 01:00


Social Studies, 26.12.2021 01:00






Biology, 26.12.2021 01:10


English, 26.12.2021 01:10

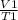 =
= 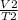
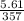 =
=