
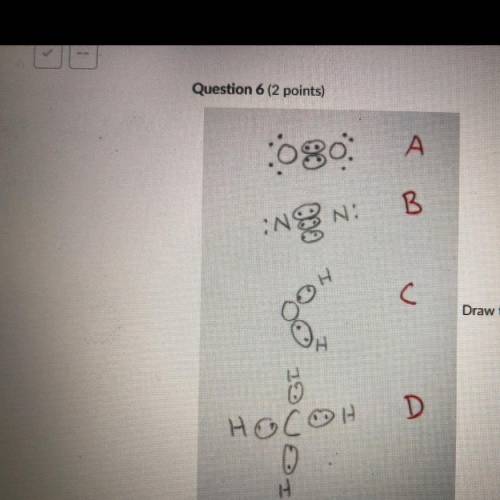
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing on this test and select
the answer that best describes which drawing is wrong and why. Note I drew circles
around electrons that are participating in covalent bonding. This is normally not done
but for the purpose of this test the circled electrons are fine.
A: O2 Is wrong because it shows the electrons at a 45 degree angle to the
Oxygen atoms.
B: N2 is wrong because it shows a triple bond.
C: H2O is wrong because it is missing 4 valence electrons.
D: CH4 is wrong because the bonds are supposed to be bent at 109.5 degrees.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing o...
Questions

Social Studies, 20.10.2019 07:00



English, 20.10.2019 07:00



History, 20.10.2019 07:00

Mathematics, 20.10.2019 07:00



English, 20.10.2019 07:00




Mathematics, 20.10.2019 07:00



History, 20.10.2019 07:00

History, 20.10.2019 07:00



