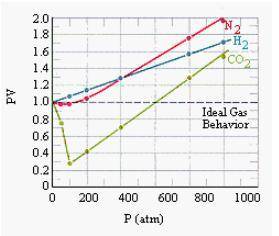
Chemistry, 17.01.2021 14:00 noahmccall647
Is 4atm to big of a difference to get when I use the ideal gas law vs the van der waals equation?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
Is 4atm to big of a difference to get when I use the ideal gas law vs the van der waals equation?...
Questions


Mathematics, 09.09.2020 22:01

Social Studies, 09.09.2020 22:01



Biology, 09.09.2020 22:01





Mathematics, 09.09.2020 22:01



Chemistry, 09.09.2020 22:01

Advanced Placement (AP), 09.09.2020 22:01


Computers and Technology, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01


History, 09.09.2020 22:01