
Chemistry, 18.01.2021 07:00 Jenniferwolf
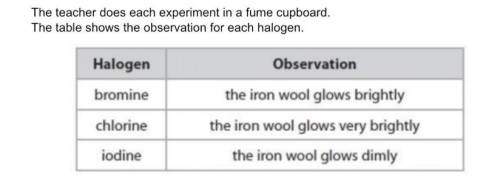
A student states that the order of reactivity cannot be found from this experiment because bromine is a liquid, chlorine is a gas and iodine is a solid at room temperature. Evaluate the student's statement.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

You know the right answer?
A student states that the order of reactivity cannot be found from this experiment because bromine i...
Questions

Business, 23.10.2019 05:00

History, 23.10.2019 05:00

History, 23.10.2019 05:00


Mathematics, 23.10.2019 05:00

Social Studies, 23.10.2019 05:00

Social Studies, 23.10.2019 05:00

Mathematics, 23.10.2019 05:00


Chemistry, 23.10.2019 05:00

English, 23.10.2019 05:00

English, 23.10.2019 05:00

Physics, 23.10.2019 05:00

Mathematics, 23.10.2019 05:00




Computers and Technology, 23.10.2019 05:00

History, 23.10.2019 05:00

History, 23.10.2019 05:00



