
Chemistry, 18.01.2021 14:00 faithb1466
The data below shows the change in concentration of dinitrogen pentoxide over time, at 330 K, according to the following process.
2N2O5(g) = 4NO2(g) + O2
[N2O5] Time (s)
0.100 0.00
0.066 200.00
0.044 400.00
a) Find the rate of disappearance of N2O5 from t=0 s to t=200s
b) Find the rate of appearance of NO2 from t=0 s to t =200s

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2


Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1
You know the right answer?
The data below shows the change in concentration of dinitrogen pentoxide over time, at 330 K, accord...
Questions

Mathematics, 01.06.2021 19:40


English, 01.06.2021 19:40


Mathematics, 01.06.2021 19:40

Mathematics, 01.06.2021 19:40





Mathematics, 01.06.2021 19:40


Mathematics, 01.06.2021 19:40



Mathematics, 01.06.2021 19:40

Mathematics, 01.06.2021 19:40

Mathematics, 01.06.2021 19:40

Physics, 01.06.2021 19:40

Mathematics, 01.06.2021 19:40

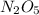 from t = 0 s to t = 200s
from t = 0 s to t = 200s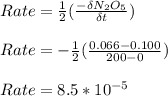
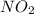 from t = 0 s to t = 200s
from t = 0 s to t = 200s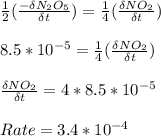
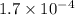
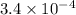
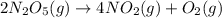
![-\frac{d[N_2O_5]}{2dt}](/tpl/images/1043/1036/c4363.png) =
= ![\frac{d[NO_2]}{4dt}](/tpl/images/1043/1036/1f481.png)
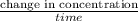 =
= 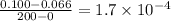
![\frac{d[NO_2]}{dt}=2\times 1.7\times 10^{-4}}=3.4\times 10^{-4}](/tpl/images/1043/1036/b438e.png)


