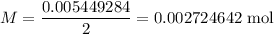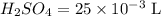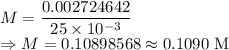Chemistry, 18.01.2021 14:00 AgarioEdit
A 25.00 mL sample of an H2SO4 solution of unknown concentration is titrated with a 0.1322 M KOH solution. A volume of 41.22 mL of KOH is required to reach the equivalence point. What is the concentration of the unknown H2SO4 solution? Express your answer in molarity to four significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
A 25.00 mL sample of an H2SO4 solution of unknown concentration is titrated with a 0.1322 M KOH solu...
Questions


History, 25.12.2019 12:31


Mathematics, 25.12.2019 12:31

Biology, 25.12.2019 12:31

Social Studies, 25.12.2019 12:31

Mathematics, 25.12.2019 12:31

History, 25.12.2019 12:31




Physics, 25.12.2019 12:31


Computers and Technology, 25.12.2019 12:31


English, 25.12.2019 12:31



Mathematics, 25.12.2019 12:31

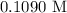
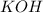 = 41.22 mL
= 41.22 mL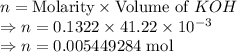
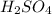
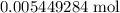 of
of