
Chemistry, 18.01.2021 16:30 21teunissen149
A group 2 metal carbonate has a mass of 84 g/mol. Identify the group 2 metal X using its chemical formula. Please show your working out. number of moles in 0.3g of calcium carbonate.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2


Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1
You know the right answer?
A group 2 metal carbonate has a mass of 84 g/mol. Identify the group 2 metal X using its chemical fo...
Questions

English, 12.12.2020 16:10


Biology, 12.12.2020 16:10




Biology, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10



Social Studies, 12.12.2020 16:10



Mathematics, 12.12.2020 16:10



Computers and Technology, 12.12.2020 16:10



Mathematics, 12.12.2020 16:10

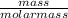
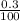 = 0.003mole
= 0.003mole

