
Chemistry, 18.01.2021 18:10 mallorytaylor279
2.In a titration, 25.00 cm3 of a solution of hydrochloric acid reacted with 18.40 cm3 of sodium hydroxide solution of concentration 0.15 mol/dm3.
The equation which represents the reaction is:
HCl + NaOH > NaCl + H2O
Calculate the concentration of hydrochloric acid in mol/dm3

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

You know the right answer?
2.In a titration, 25.00 cm3 of a solution of hydrochloric acid reacted with 18.40 cm3 of sodium hydr...
Questions

Social Studies, 29.10.2020 21:00

Arts, 29.10.2020 21:00

Computers and Technology, 29.10.2020 21:00



English, 29.10.2020 21:00


Mathematics, 29.10.2020 21:00

Spanish, 29.10.2020 21:00

Mathematics, 29.10.2020 21:00




Mathematics, 29.10.2020 21:00

Mathematics, 29.10.2020 21:00

Business, 29.10.2020 21:00

History, 29.10.2020 21:00


Mathematics, 29.10.2020 21:00

SAT, 29.10.2020 21:00

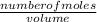 =
= 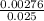 = 0.11mol/dm³
= 0.11mol/dm³

