

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Iodine monochloride (ICl) has a higher boiling point than bromine (Br2) partly because iodine monoch...
Questions

Mathematics, 21.10.2021 01:00

Mathematics, 21.10.2021 01:00




English, 21.10.2021 01:00





Chemistry, 21.10.2021 01:00


Advanced Placement (AP), 21.10.2021 01:00


Mathematics, 21.10.2021 01:00


Mathematics, 21.10.2021 01:00

History, 21.10.2021 01:00

Computers and Technology, 21.10.2021 01:00

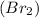 is a non polar molecule as there is no electronegativity difference between two bromine atoms. The molecules are bonded by weak vanderwaal forces and thus has low boiling point.
is a non polar molecule as there is no electronegativity difference between two bromine atoms. The molecules are bonded by weak vanderwaal forces and thus has low boiling point.

