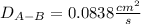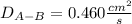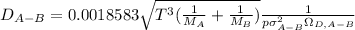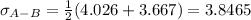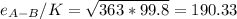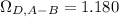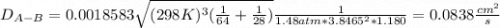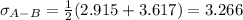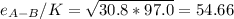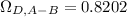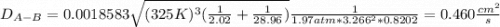Chemistry, 18.01.2021 21:10 KittyLoverCat
Estimate the value of the gas-phase diffusion coefficient for the following pairs using the Hirshfelder equation: a. Sulfur dioxide and nitrogen (N2) at 298 K and 1.5 x 105 Pa b. Hydrogen (H2) and air at 325 K and 2.0 x 105 Pa

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3


Chemistry, 23.06.2019 08:40
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1
You know the right answer?
Estimate the value of the gas-phase diffusion coefficient for the following pairs using the Hirshfel...
Questions

Computers and Technology, 24.06.2021 19:10


Chemistry, 24.06.2021 19:10


Health, 24.06.2021 19:10

Mathematics, 24.06.2021 19:10

Mathematics, 24.06.2021 19:10

Mathematics, 24.06.2021 19:10

Mathematics, 24.06.2021 19:10





Mathematics, 24.06.2021 19:10



Health, 24.06.2021 19:10

Mathematics, 24.06.2021 19:10

Health, 24.06.2021 19:10

Computers and Technology, 24.06.2021 19:10