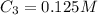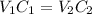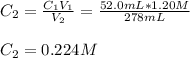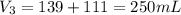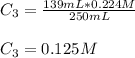Chemistry, 18.01.2021 21:10 tylermorehead1
A 52.0 mL portion of a 1.20 M solution is diluted to a total volume of 278 mL. A 139 mL portion of that solution is diluted by adding 111 mL of water. What is the final concentration

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
A 52.0 mL portion of a 1.20 M solution is diluted to a total volume of 278 mL. A 139 mL portion of t...
Questions


Chemistry, 14.07.2021 14:00


Mathematics, 14.07.2021 14:00

English, 14.07.2021 14:00

History, 14.07.2021 14:00


English, 14.07.2021 14:00

Biology, 14.07.2021 14:00


Social Studies, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00


Mathematics, 14.07.2021 14:00



Mathematics, 14.07.2021 14:00


Mathematics, 14.07.2021 14:00