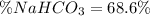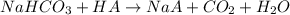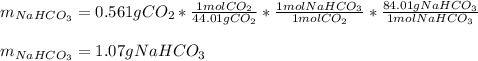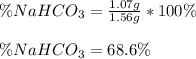Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

You know the right answer?
A mixture contains N a H C O 3 together with unreactive components. A 1.56 g sample of the mixture r...
Questions


Health, 19.12.2019 17:31






English, 19.12.2019 17:31

Mathematics, 19.12.2019 17:31


Mathematics, 19.12.2019 17:31


Social Studies, 19.12.2019 17:31


Mathematics, 19.12.2019 17:31


Mathematics, 19.12.2019 17:31

Mathematics, 19.12.2019 17:31


Mathematics, 19.12.2019 17:31