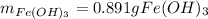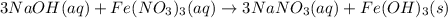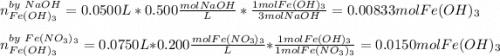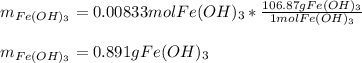Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

You know the right answer?
How many grams of iron(III) hydroxide (106.87 g/mol) will precipitate if 50.0 mL of 0.500 M sodium h...
Questions


Mathematics, 25.10.2020 23:20

Mathematics, 25.10.2020 23:20

Spanish, 25.10.2020 23:20




Computers and Technology, 25.10.2020 23:20

Mathematics, 25.10.2020 23:20

History, 25.10.2020 23:20

Physics, 25.10.2020 23:20

Biology, 25.10.2020 23:20

Mathematics, 25.10.2020 23:20

History, 25.10.2020 23:20

Mathematics, 25.10.2020 23:20



Biology, 25.10.2020 23:20


History, 25.10.2020 23:20