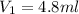Chemistry, 18.01.2021 21:40 lkarroum3733
Calculate the volume in mL of 1.043 M acetic acid (CH3COOH) that is needed to prepare 50.0 mL of 0.10 M acetic acid. Show your work and give your answer to the correct number of significant figures.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Calculate the volume in mL of 1.043 M acetic acid (CH3COOH) that is needed to prepare 50.0 mL of 0.1...
Questions




Mathematics, 08.04.2021 01:10

Mathematics, 08.04.2021 01:10

Mathematics, 08.04.2021 01:10


Mathematics, 08.04.2021 01:10

Chemistry, 08.04.2021 01:10


Mathematics, 08.04.2021 01:10

Mathematics, 08.04.2021 01:10

Mathematics, 08.04.2021 01:10



History, 08.04.2021 01:10

Advanced Placement (AP), 08.04.2021 01:10

Mathematics, 08.04.2021 01:10

Business, 08.04.2021 01:10

Mathematics, 08.04.2021 01:10

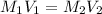
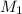 = molarity of stock
= molarity of stock 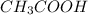 solution = 1.043 M
solution = 1.043 M
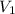 = volume of stock
= volume of stock