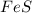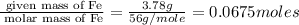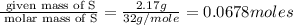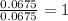Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1

Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
In a reaction, 3.78g of iron metal is reacted completely with excess sulfur to produce only one prod...
Questions

Mathematics, 06.11.2020 18:50

Mathematics, 06.11.2020 18:50

Mathematics, 06.11.2020 18:50

Chemistry, 06.11.2020 18:50

Social Studies, 06.11.2020 18:50


Mathematics, 06.11.2020 18:50


English, 06.11.2020 18:50




English, 06.11.2020 18:50

Mathematics, 06.11.2020 18:50



Mathematics, 06.11.2020 18:50

Mathematics, 06.11.2020 18:50

History, 06.11.2020 18:50