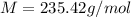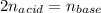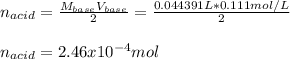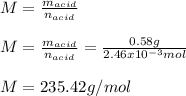Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2


Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
An unknown diprotic acid (H2A) requires 44.391 mL of 0.111 M NaOH to completely neutralize a 0.58 g...
Questions

Mathematics, 08.04.2021 20:40

Mathematics, 08.04.2021 20:40

Advanced Placement (AP), 08.04.2021 20:40

Mathematics, 08.04.2021 20:40


Chemistry, 08.04.2021 20:40

History, 08.04.2021 20:40

Mathematics, 08.04.2021 20:40

Geography, 08.04.2021 20:40

Mathematics, 08.04.2021 20:40

Mathematics, 08.04.2021 20:40



Mathematics, 08.04.2021 20:40




History, 08.04.2021 20:40

Mathematics, 08.04.2021 20:40