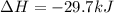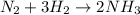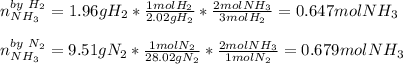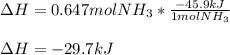Chemistry, 19.01.2021 01:00 browneyedbaby20
The standard molar enthalpy of formation of NH3(g) is -45.9 kJ/mol. What is the enthalpy change if 9.51 g N2(g) and 1.96 g H2(g) react to produce NH3(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1
You know the right answer?
The standard molar enthalpy of formation of NH3(g) is -45.9 kJ/mol. What is the enthalpy change if 9...
Questions

History, 29.11.2021 19:00

Mathematics, 29.11.2021 19:00

Mathematics, 29.11.2021 19:10

Mathematics, 29.11.2021 19:10



Computers and Technology, 29.11.2021 19:10


Physics, 29.11.2021 19:10

Mathematics, 29.11.2021 19:10

English, 29.11.2021 19:10


Mathematics, 29.11.2021 19:10

Social Studies, 29.11.2021 19:10


Mathematics, 29.11.2021 19:10

Social Studies, 29.11.2021 19:10

Social Studies, 29.11.2021 19:10