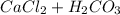Chemistry, 19.01.2021 03:20 bellbradshaw16
Aqueous solutions of Na2CO3 and Ca(NO3)2, 0.10 M each, are combined. A white precipitate is observed in the container after mixing. The precipitate is filtered and carefully rinsed with distilled water to remove other ions. A sample of the precipitate is added to 100 mL of 0.1 M NaCl. A second sample of the precipitate is then added to 100 mL of 0.1 M HCl. What would be observed in each case

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1

Chemistry, 23.06.2019 12:00
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
You know the right answer?
Aqueous solutions of Na2CO3 and Ca(NO3)2, 0.10 M each, are combined. A white precipitate is observed...
Questions


English, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

History, 30.08.2019 04:30







English, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Biology, 30.08.2019 04:30

English, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Computers and Technology, 30.08.2019 04:30

Biology, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

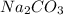 and
and 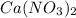 , 0.10 M each, are combined. A white precipitate is observed in the container after mixing. he precipitate is filtered andcarefully rinsed with distilled water to remove other ions. A sample of the precipitate is added to 100 mL of 0.1 M NaCl. A second sample of the precipitate is then added to 100 mL of 0.1 M HCl. What would be observed in each case?
, 0.10 M each, are combined. A white precipitate is observed in the container after mixing. he precipitate is filtered andcarefully rinsed with distilled water to remove other ions. A sample of the precipitate is added to 100 mL of 0.1 M NaCl. A second sample of the precipitate is then added to 100 mL of 0.1 M HCl. What would be observed in each case?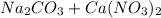 →
→ 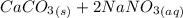
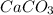 ), which, as it is insoluble in water, precipitates as a solid of the color white. This process is Precipitation and this reaction is a Precipitation Reaction.
), which, as it is insoluble in water, precipitates as a solid of the color white. This process is Precipitation and this reaction is a Precipitation Reaction. 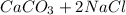 →
→ 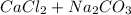
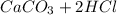 →
→