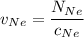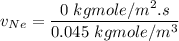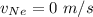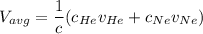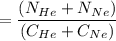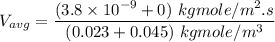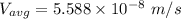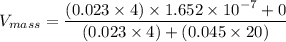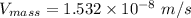Chemistry, 19.01.2021 05:30 Jadamachado45
In a gas-phase diffusion mass-transfer process, the steady-state flux of helium in a binary mixture of helium and neon is 3.8 x 10-9 kgmole/cm2 s, and the flux of neon is 0. At a particular point in the diffusion space, the concentration of helium is 0.023 kgmole/m3 and the concentration of neon is 0.045 kgmole/m3 . Estimate the individual net velocities of helium and neon along the direction of mass transfer, the average molar velocity, and the average mass velocity.''

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
In a gas-phase diffusion mass-transfer process, the steady-state flux of helium in a binary mixture...
Questions

History, 20.12.2019 01:31

Mathematics, 20.12.2019 01:31

Mathematics, 20.12.2019 01:31

History, 20.12.2019 01:31

History, 20.12.2019 01:31

Health, 20.12.2019 01:31



Mathematics, 20.12.2019 01:31


Biology, 20.12.2019 01:31

Mathematics, 20.12.2019 01:31

Mathematics, 20.12.2019 01:31



History, 20.12.2019 01:31



English, 20.12.2019 01:31

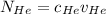
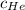 = concentration of Helium
= concentration of Helium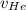 = net velocity of Helium
= net velocity of Helium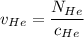
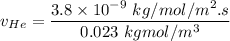
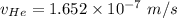
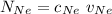
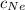 = Concentration of neon
= Concentration of neon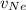 = net velocity of neon species
= net velocity of neon species