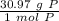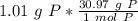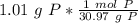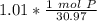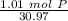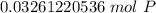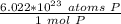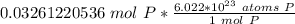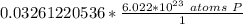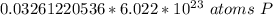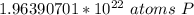Chemistry, 19.01.2021 06:00 TH3L0N3W0LF
How many atoms are present in a 1.01g sample of Phosphorus (P)? [Note: 1 mol = 6.022x10²³ atoms, molar mass of Phosphorus =30.97 g/mol) *
1 point
Captionless Image
3.48 x 10²¹ atoms
1.97 x 10²² atoms
1.04 x 10²⁶ atoms
3.36 x 10²⁴ atoms

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
How many atoms are present in a 1.01g sample of Phosphorus (P)? [Note: 1 mol = 6.022x10²³ atoms, mol...
Questions







Mathematics, 10.03.2020 00:43





Business, 10.03.2020 00:43




Mathematics, 10.03.2020 00:43


Biology, 10.03.2020 00:43