How many moles of NO2 are there in 5.10 x 10 18*molecules of NO2?
...

Chemistry, 19.01.2021 08:50 abadir2008
How many moles of NO2 are there in 5.10 x 10 18*molecules of NO2?
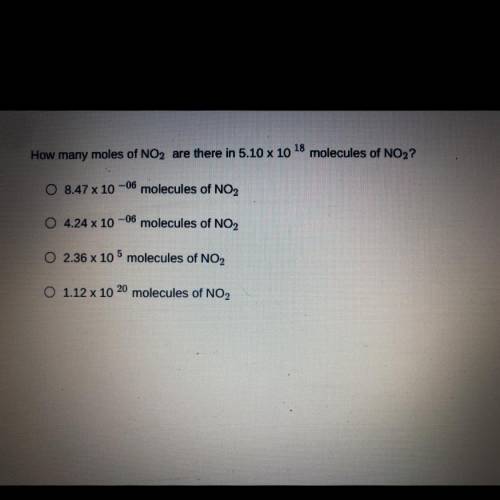

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1


Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2
You know the right answer?
Questions

Chemistry, 06.06.2021 14:00

Biology, 06.06.2021 14:00




Chemistry, 06.06.2021 14:00

Social Studies, 06.06.2021 14:00


Arts, 06.06.2021 14:00


Mathematics, 06.06.2021 14:00


History, 06.06.2021 14:00


Mathematics, 06.06.2021 14:00


Social Studies, 06.06.2021 14:00

Mathematics, 06.06.2021 14:00


Mathematics, 06.06.2021 14:00



