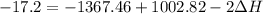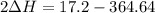Chemistry, 19.01.2021 19:40 supergraciepie
A scientist measures the standard enthalpy change for the following reaction to be -17.2 kJ : Ca(OH)2(aq) 2 HCl(aq)CaCl2(s) 2 H2O(l) Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of HCl(aq) is kJ/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
A scientist measures the standard enthalpy change for the following reaction to be -17.2 kJ : Ca(OH)...
Questions



Business, 23.01.2020 18:31

English, 23.01.2020 18:31



English, 23.01.2020 18:31

History, 23.01.2020 18:31




Mathematics, 23.01.2020 18:31

Biology, 23.01.2020 18:31

Physics, 23.01.2020 18:31

Biology, 23.01.2020 18:31

History, 23.01.2020 18:31

Business, 23.01.2020 18:31

Mathematics, 23.01.2020 18:31

Mathematics, 23.01.2020 18:31

Mathematics, 23.01.2020 18:31

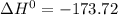 kJ/mol
kJ/mol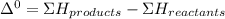
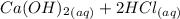 →
→ 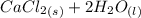
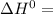 -1002.82 kJ/mol
-1002.82 kJ/mol![-17.2=[-795.8+2(285.85)]-[-1002.82+2\Delta H]](/tpl/images/1045/5683/27b56.png)