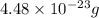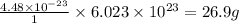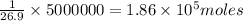An aluminum atom has a mass of 4.48 * 10-23 g and a small airplane has a mass of 5000 kg. Use this information to answer the questions below. Be sure your answers have the correct number of significant digits.
What is the mass of 1 mole of aluminum atoms?
Round your answer to 3 significant digits.
g
How many moles of aluminum atoms have a mass equal to the mass of a small airplane?
Round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

You know the right answer?
An aluminum atom has a mass of 4.48 * 10-23 g and a small airplane has a mass of 5000 kg. Use this i...
Questions


Arts, 03.03.2021 03:20

English, 03.03.2021 03:20

Biology, 03.03.2021 03:20






Mathematics, 03.03.2021 03:20








History, 03.03.2021 03:20

Health, 03.03.2021 03:20

Mathematics, 03.03.2021 03:20

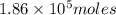
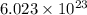 of particles.
of particles.