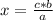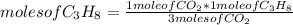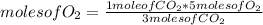Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2

You know the right answer?
C3H8 (g) 5O2 (g) -> 3CO2 (g) 4H2O (l) in the reaction represented baove, what is the total number...
Questions


English, 22.05.2021 01:00

Mathematics, 22.05.2021 01:00


Mathematics, 22.05.2021 01:00





Mathematics, 22.05.2021 01:00

Mathematics, 22.05.2021 01:00


Mathematics, 22.05.2021 01:00


Mathematics, 22.05.2021 01:00


Mathematics, 22.05.2021 01:00


Chemistry, 22.05.2021 01:00